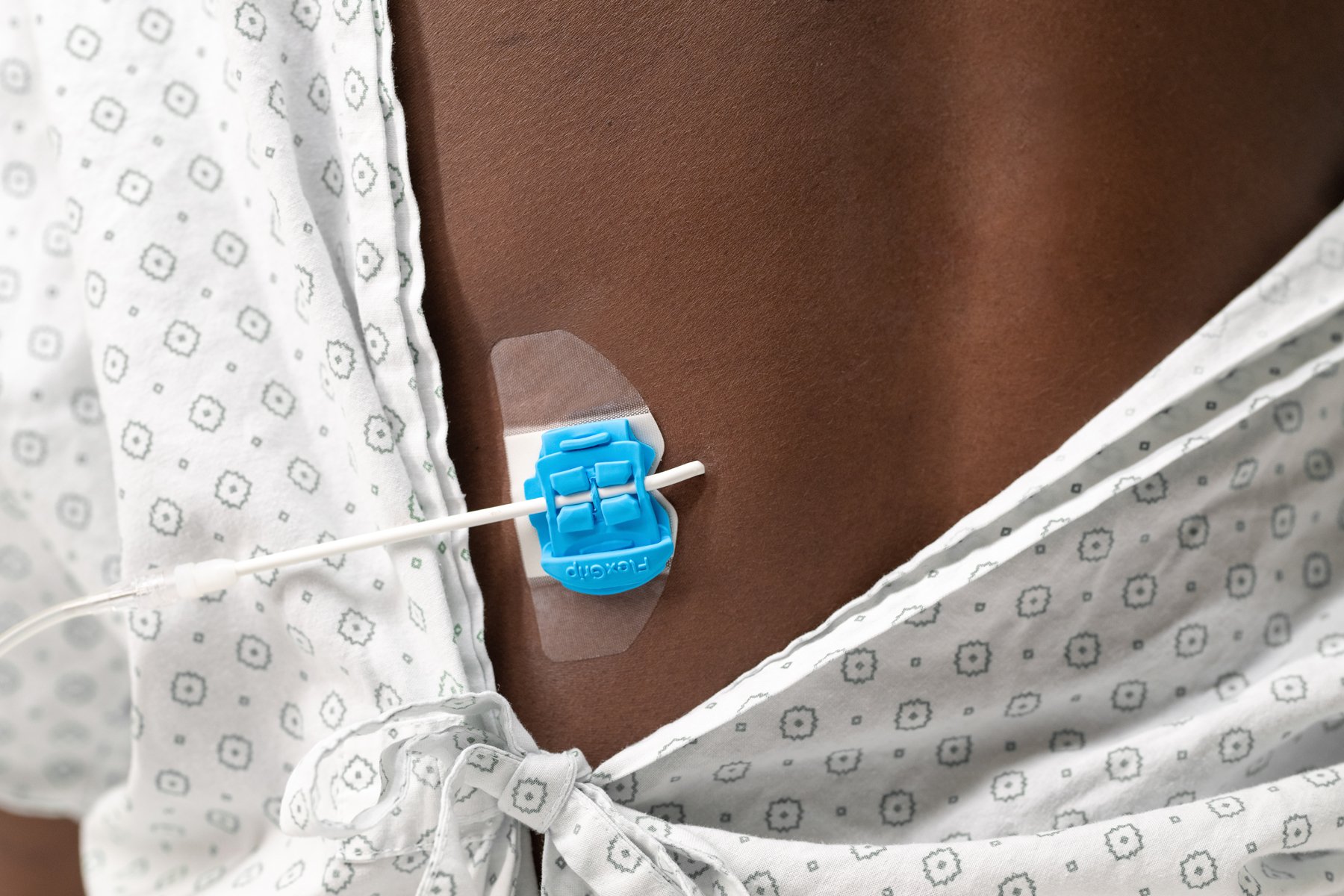
Catheters can be a pain. Literally. Bedal’s FlexGRIP products strongly improve catheter stabilization—reducing the risk of dislodgement, infection, and kinking. Bonus: FlexGRIP makes for a much more comfortable experience.
catheter stabilization,
the smart way.

catheter stabilization,
the smart way.
Catheters can be a pain. Literally. Bedal’s FlexGRIP products strongly improve catheter stabilisation—reducing the risk of dislodgement, infection, and kinking. Bonus: FlexGRIP makes for a much more comfortable experience.
“FlexGRIP is vastly superior to any other stabilization device I have ever encountered “
Steve Bierman MD
Former founder and CEO of Statlock
Inventor of +200 patents on stabilization devices
FlexGRIP is…
comfortable
Focusing on patient comfort, we used flexible material that moves along with the skin. This causes less discomfort and allows the patient to move more freely.
user-friendly
We developed a specific technique for quick and easy placement. Beneficial for healthcare professionals, but also for patients undergoing the procedure.
safe
The primary function of any catheter stabilization device? Ensuring secure attachment. Good thing FlexGRIP is about twice as strong as regular catheter stabilizers.
flexible
In terms of material, yes, but our patches are also widely applicable. A wide range of catheters can be stabilized with a minimal amount of products.
get in touch
Do you have a question for us? Want to know more about one of our products or have an inquiry? Please get in touch. We're here to help, and we would love to tell you more about FlexGRIP and all of its benefits.